Soda lime is responsible for the elimination of carbon dioxide (CO2) in rebreathing circuits. When exhausted, CO2 accumulates in the circuit and is rebreathed by the patient, causing respiratory acidosis that can be harmful.
Key points: methods to know when to change soda lime
- Increased inspired CO2 (detected by capnography)
- Color change
- Lack of heat in the canister
- Clinical signs
- Hardness of granules
- Time in function
Measuring inspired CO2
Measuring inspired CO2 is the most reliable method to detect absorbent exhaustion. This can be done with a capnograph. A typical waveform obtained during CO2 reinspiration caused by soda lime exhaustion is illustrated in Figure 1. During inspiration, the patient breathes CO2: the curve does not return to 0 during inspiration. The shape of the trace is normal, and the end-tidal CO2 value is increased.
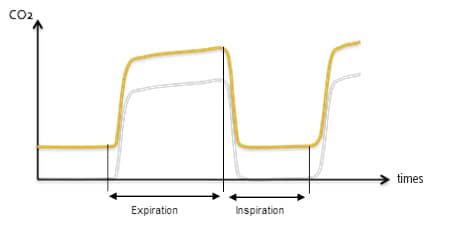
Figure 1: Typical capnograph waveform obtained when soda lime is exhausted (yellow), compared to a normal waveform (grey). During inspiration, the curve does not return to 0, but the shape of the waveform is normal. End-tidal CO2 value is increased.
Indicator color change
When CO2 reacts with soda lime, heat and water are formed, and pH changes. The latter causes the indicator contained in soda lime to change color (typically from white to pink), indicating that the absorbent is near the point of exhaustion.
Absorbent should be changed when 2/3 of the canister has changed color. Keep in mind that the color may revert back to its pre-exhaustion color when not in use. Upon reuse, the indicator color will rapidly return to it’s exhausted state. Therefore, a rested canister can give a false sense of security. For this reason, inspection of the absorbent color should be made during or just after anesthesia.
Additionally, if the absorbent is not packed properly in the canister, channeling can occur: the airflow passes through a channel in the soda lime, exposing only a small part of the absorbent to CO2. As absorbent along the channel becomes exhausted quickly, the patient rebreathes CO2. The rest of the soda lime remains white, giving a false sense of security. Figure 2 illustrates those patterns of absorption.
Therefore, indicator color change is useful but is not reliable.
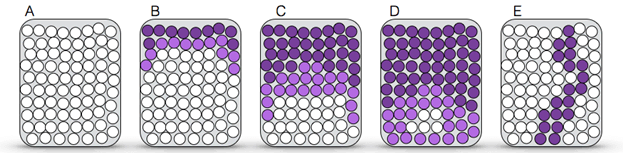
Figure 2: Pattern of CO2 absorption in the canister. Purple circles represent exhausted soda lime; white circles represent fresh soda lime.
- A) Unused canister, or appearance of the canister after some time unused: all granules are white.
- B) After limited use: absorption of CO2 has occurred primarily at the inlet and to a lesser extent along the sides.
- C) After extensive use: the canister appears nearly completely purple as granules on the side are exhausted.
- D) Exhausted soda lime: CO2 is filtering through the canister; the only granules that are still capable of absorbing CO2 are in the distal third.
- E) Channeling effect: air passes through the soda lime preferentially through a channel. Soda lime, in this channel, is quickly exhausted, and the patient breathes CO2 even though the canister remains white if the channel is not along the wall.

